In the past 10 years (2011-2021), the number of diabetes patients in China has grown from 90 million to 140 million, representing an increase of 56%. The huge medical expenditure on diabetes brings a very heavy burden to patients, families and society as a whole. Diabetes is mainly divided into Type 1 and Type 2 diabetes due to different pathogenesis. As the disease progresses, the patient’s pancreatic islets β cells experience a significant decrease leading to the absolute deficiency of insulin secretion. Therefore, in addition to the basic exogenous insulin injection therapy, a suitable β Cell replacement therapy has become a fundamental treatment for diabetes. At present, islet allografts can effectively treat diabetes. However, due to the lack of donors, there is a serious mismatch between supply and demand. While human embryonic stem cells induce differentiation into pancreatic islets human embryonic stem cell derived β cell (SC- β cell) technology brings new hope for the treatment of diabetes through quantitative advantages.
The academic community has reached a consensus on the cutting-edge technology of differentiation of human pluripotent stem cells into islets β cells that there are two bottlenecks restricting its development: (i) poor functional maturity, unable to respond well to glucose stimulation; (ii). vulnerability and low survival rate beyond short periods under the metabolic pressure of high fat and sugar in diabetic patients. It follows that there is an urgent challenge to acquire islets β cells that are more mature and can function longer and in a more stable manner. For a long time, improvement, by gene editing β cell function and survival, has been the focus of contemporary academic research, but there has been no substantive progress made yet. One of the most important factors for this has been the lack of suitable targets.
On July 16, Professor LI Weida's research group and their collaborators from the School of Life Science and Technology of Tongji University, the Institute of Regenerative Medicine of the Oriental Hospital Affiliated to Tongji University, and the Frontier Science Center of "Cell Stem and Fate Editing" of the Ministry of Education published a paper in Nature Communications entitled "ZnT8 loss of function accelerates functional matrix of hESC derived" β Cells and resists metabolic stress in diabetes”. This study targeted the SLC30A8, functional inactivation reduces the risk of human diabetes, gene, knocked out the gene in stem cells, and induced β cell differentiation to enhance cell function and life expectancy of the SC-β cells. has overcome the two bottlenecks and promoted the development of cell replacement therapy for diabetes.

Article published in Nature Communications
The zinc ion transporter ZnT8 encoded by the SLC30A8 gene is an islet-specific zinc transporter, which is highly expressed in β cells secrete vesicles. These are responsible for transporting zinc ions from the cytoplasm into insulin secretion vesicles. Of interest, the study found that SC lacking ZnT8 protein- βcells always showed robust insulin secretion capacity under the stimulation of higher concentrations of glucose (20 mM). Further studies showed that ZnT8 was only expressed in SC- β, the mature stage of cell differentiation and development, i.e. in S6 and S7 stages, which means that ZnT8 may be involved in β cell regulation of functional development and maturation. Single cell sequencing showed that in ZnT8 knockout in SC- βcells, β functional related genes such as INS, NKX6.1, PDX1, SYT13 and VAMP2 significantly increased compared with those in the control group. Simulated temporal analysis also showed that development of SC-β cells with deficient ZnT8 in the last two stages of differentiation is more mature.
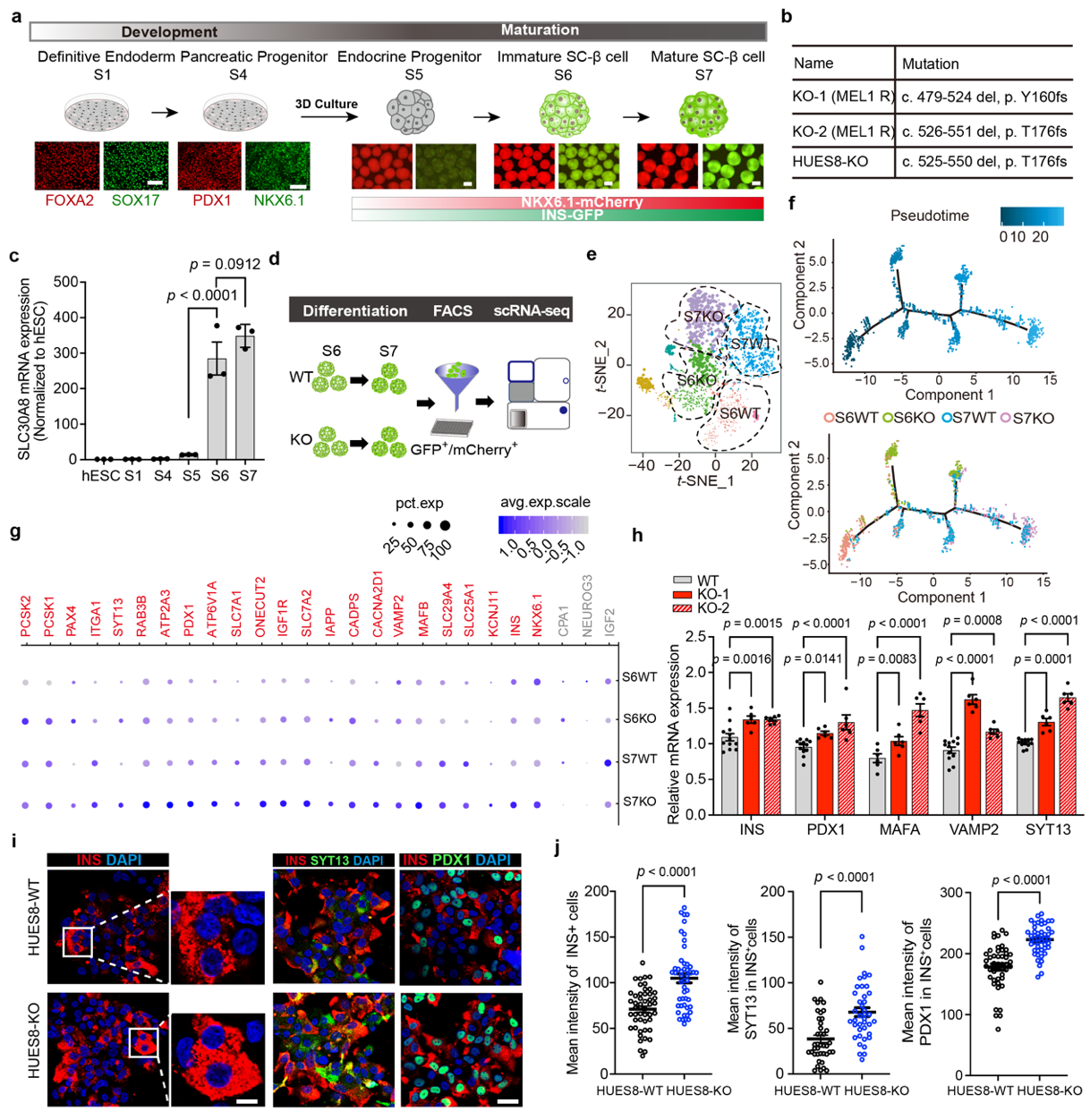
It was discovered that SC- β Cells with deficient ZnT8 protein always exhibit robust insulin secretion under stimulation of higher concentrations of glucose (20 mM)
A glucose stimulated insulin secretion (GSIS) experiment is an optimal approach to test SC-β cell function. The multiple of insulin secretion under high glucose stimulation compared with that under low glucose stimulation, can directly reflect how β cells respond to sugar stimulation: the Higher, the stronger. In order to verify the above results, the researchers conducted GSIS experiments in three independent ZnT8 knockout cells. They found that SC- β cells with deficient ZnT8 under two rounds of low glucose high glucose stimulation showed a phenotype of enhanced insulin secretion compared to wild-type SC- β cells. Through further exploration, researchers found that the insulin secretion of ZnT8 deficient SC- β cells strong may be related to the release of negative feedback inhibition of zinc ion on insulin secretion.
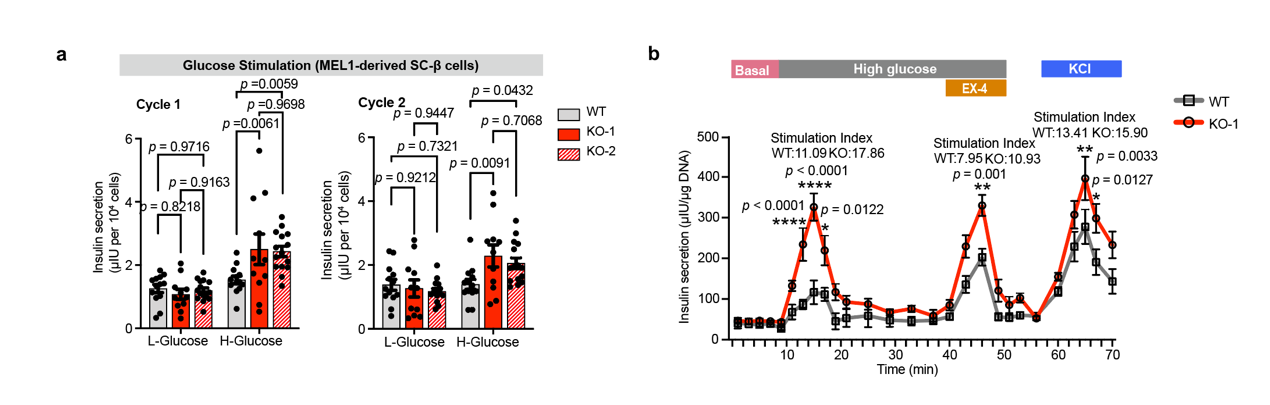
The glucose stimulated insulin secretion (GSIS) experiment
This study found that SC- β cells with deficient ZnT8, the number of apoptotic cells in the cells treated with lipotoxic inducers such as palmitic acid was significantly less than that in the control group under the same treatment, which means thatβ cells ZnT8 deleted can better resist metabolic stress caused by high fat. Further analysis revealed in the case that ZnT8 deficient SCS- β, under the stimulation of certain concentrations of palmitic acid, the cells did not show marked increase of ER stress markers represented by IRE1α and sXBP1 as those in the control group, and the level of intracellular calcium ion and mitochondrial depolarization, were also significantly lower than those in the control group. The above results indicate that ZnT8 deficiency can resist lipotoxicity stress on SC- β cells of the endoplasmic reticulum caused by cells, thereby reducing apoptosis.
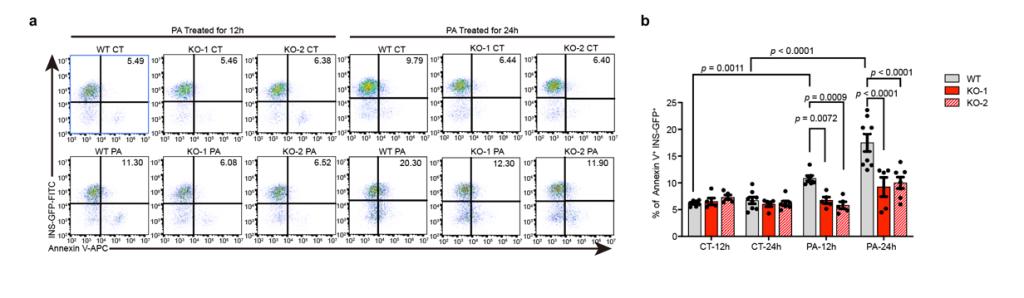
ZnT8 deficiency can resist lipotoxicity stress on SC- β cells of the endoplasmic reticulum caused by cells, thereby reducing apoptosis
The researchers transplanted ZnT8 deficient SCS- β cells and those of the control group into the renal capsule of SCID-Beige immunodeficient mice modeled with streptozotocin (STZ), and the blood glucose changes of the mice were continuously monitored after transplantation. The researchers found that with transplanted ZnT8 deficient SC- β cells, after a period of transplantation, the blood glucose of the mice with the cells decreased more rapidly and the extent of this decrease was larger. When the blood glucose dropped below 180 mg / dL, the blood glucose of the mice was considered to be normal. By calculating the cure rate, it can be found that the cure rate of diabetes in mice with transplanted wild-type SC-β cells was 42.9%, and cure rate of diabetes in mice with SC-β cells of ZnT8 function loss was transplanted at the same dose was 73.3%, with the latter 30.4% higher than the former. Cells can survive in a more stable condition in mice with genetically edited SC- SC- β cells compared to unedited SCS- β cells.
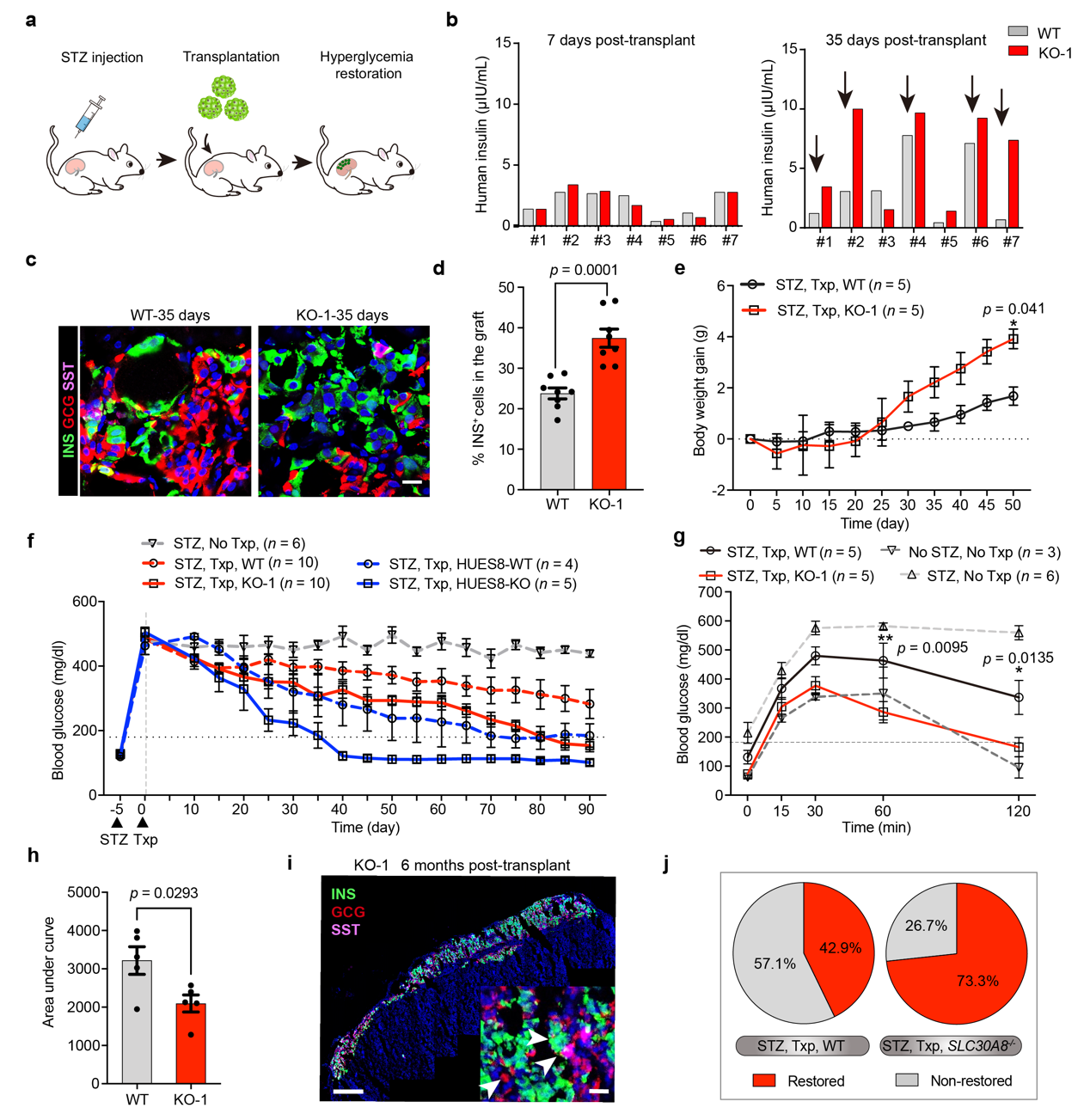
The researchers transplanted ZnT8 deficient SC- β Cells and those of the control group into the renal capsule of SCID-Beige immunodeficient mice modeled with streptozotocin (STZ)
At present, clinical trials of β-cell replacement therapy of stem cells induced differentiation into β cells were performed for patients with diabetes in the United States. The results of clinical trials Phases 1 and 2 by Certex Company showed that the first transplant patient recovered basic insulin production on the 90th day after transplantation, and the daily demand for exogenous insulin was reduced by 91%. On Day 270 of transplantation, the patient was completely weaned from exogenous insulin use. Although the above clinical trials have achieved good results, the effect of SC- β cells were slow, which proves that the functions of the cells themselves are immature and need to rely on the internal environment for further development and maturation, and the long-term stability of the cells in the diabetes environment remains to beseen. In this study, ZnT8 protein was inactivated and successfully enhanced β cell function and improved βcells in resisting metabolic resistance to accelerates the recovery of blood glucose in mice with diabetes after transplantation. It improves the cure rate of diabetes in mice, and optimizes diabetes β cell replacement therapy, thus helping develop new targets and methods.
Professor LI Weida's research group focused their research on "β cell regeneration in diabetes” and proposed a high-efficiency pancreatic trans-differentiation system to improve the trans-differentiation of pancreatic exocrine cells into pancreatic islets β cells efficiency. Functional human islet organoids with resistance to the pathological environment of diabetes were also constructed by gene editing, and a more efficient and stable therapeutic effect was achieved in the animal model of diabetes. New mechanism of islets β cells in diabetes was analyzed. Their research results have been published in well-known academic journals such as Nature Biotechnology, Nature Communications, eLife and Advanced Materials. Invention patents have also been applied for. At the same time, Professor LI Weida's team cooperated closely with clinical departments, such as the Metabolism Department and Endocrinology Department, to apply this technology to the clinical transformation of diabetes stem cell medicine.
The co corresponding authors of the paper were: Professor LI Weida, researcher CHENG Xin of the Center of Excellence and Innovation in Molecular Cell Science of the Chinese Academy of Sciences, and Professor DENG Qiaolin of Karolinska College of Sweden. MA Qing, a doctoral student of Tongji University, XIAO Yini, doctoral student of the Center for Excellence and Innovation in Molecular and Cell Sciences of the Chinese Academy of Sciences, and XU Wenjun, doctoral student of Tongji University, are the co first authors of this paper. The research was supported by the 2016 Key Research and Development Plan of the Ministry of Science and Technology of the People's Republic of China, Stem Cell and Transformation Special Youth Project with Professor LI Weida as the chief scientist, and also by the National Natural Science Foundation of China and Shanghai Municipal Science and Technology Commission.