On the evening of July 7, the team comprising GAO Shaorong, LIU Xiaoyu and WANG Chenfei from the School of Life Science and Technology of Tongji University and the team of OU Xianghong from the Second People's Hospital of Guangdong Province published an online cover article entitled "Stage-specific H3K9me3 occupancy ensures retrotransposon silencing in human preimplantation embryos" in Cell Stem Cell. The article describes the dynamic change map of histone H3K9me3 modification during the development of human preimplantation embryos; reveals the establishment and function of stage specific H3K9me3 modification on retrotransposons; and discovers the key regulatory factors that may mediate the establishment of H3K9me3 modification.

The article published in Cell Stem Cell
As an introduction, GAO observed that histone H3K9me3 modification, as one of the markers of heterochromatin, plays a very important role in mammalian embryonic development and cell fate determination. The incomplete removal of H3K9me3 is the key reason that the development of somatic cloned embryos is blocked during the zygotic genome activation (ZGA) period, and the premature establishment of heterochromatin enriched in H3K9me3 will also seriously hinder the development of preimplantation embryos in mice. In the ZGA stage of humans and mice (humans are mainly at the 8-cell stage and mice are mainly at the 2-cell stage), a large number of transposons are actively transcribed, including MERVL / HERVL, members of long terminal repeats (LTR). Because the transposition and self-replication ability of LTR bring potential harm to the integrity of mammalian genome, its expression must be strictly regulated. However, due to the limitation of histone modification detection technology and the scarcity of human preimplantation embryo materials, the reprogramming mechanism and function of H3K9me3 modification in human early embryo development are not very clear.
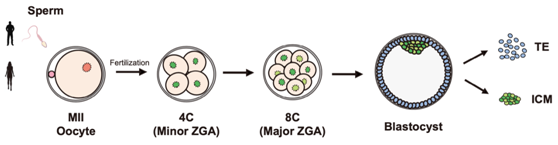
Fig. 1 Schematic diagram of human preimplantation embryo sample collection
In order to explore the dynamic changes of H3K9me3 modification during the development of human preimplantation embryos, researchers collected human MII oocytes, 4-cell, 8-cell and blastocyst embryos, and used CUT & RUN technology to detect the H3K9me3, H3K4me3 and H3K27me3 modification levels at various stages (Fig. 1). As expected, H3K9me3 modification was also highly enriched in the LTR region in human early embryos, and heterochromatin enriched in H3K9me3 was gradually established (Fig. 2). In addition, there is a relatively conserved mechanism of LTR silencing mediated by H3K9me3 in human early embryos compared with mice.
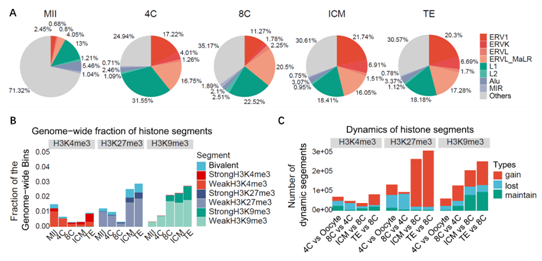
Fig. 2 Distribution and dynamic changes of H3K9me3 modification on genome
Researchers found that the establishment of H3K9me3 modification on the LTR region in human early embryos is time-specific, which ensures the orderly silencing of LTR families expressed at different embryonic stages. Interestingly, the analysis results showed that the H3K9me3 modification temporarily established in human 8-cells was more likely to enrich H3K4me1 and H3K27ac modifications at the subsequent development stage, indicating that its modified region might be transformed into an enhancer at a later stage, while the H3K9me3 modification established in blastocysts was more stable (Fig. 3).
In order to study how the establishment of period specific H3K9me3 modification is regulated, the researchers predicted the transcription factors that may participate in this regulation according to the binding sites of transcription factors and the distribution of H3K9me3 modification. The results showed that some classical regulatory factors related to H3K9me3 modification (including TRIM28 and KRAB ZNFs) may participate in the regulation of blastocyst specific H3K9me3 labeled LTR, while the activating transcription factor DUX4 and other KRAB ZNFs may be involved in the regulation of 8-cell-specific H3K9me3 labeled LTR (Figure 3).
Since it is impossible to directly verify the function of these regulatory factors in human embryos, the analysis found that there is also a highly conserved period specific regulation of H3K9me3 modification in the early embryonic development of mice. The researchers selected the homologues of these candidate factors in mice for analysis. They found that deletion of Dux affects the establishment of cleavage stage specific (corresponding to human 8-cell specific) and blastocyst stage specific H3K9me3 modifications. On the other hand, after siRNA injection in mice fertilized eggs to reduce Zfp51 in embryos, researchers found that lack of Zfp51 significantly affected the establishment of H3K9me3 on blastocyst specific LTR, while other LTRs were not affected.
The results show that both Dux and Zfp51 can regulate the establishment of specific H3K9me3 in the LTR region of the early embryos of mice, and the precise regulation of LTR silencing is important for normal embryonic development. The study also revealed that the bivalent domain of H3K4me3 / H3K9me3 was gradually established during the development of human preimplantation embryos, which may be similar to the bivalent domain of H3K4me3 / H3K27me3 and play a role in regulating lineage differentiation.
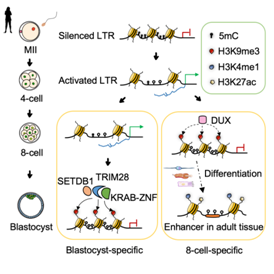
Fig. 3 Schematic diagram of LTR regulation mechanism of H3K9me3 marker during human early embryonic development
GAO observed that this study revealed the process of H3K9me3 modification and reprogramming in the process of ZGA, the first lineage differentiation of human embryos, and described the rule of heterochromatin remodeling in human preimplantation embryo development. In addition, this study proposed that activated DUX4 has an important contribution to the establishment of inhibitory histone H3K9me3 modification in human ZGA stage, which provides new insights for a better understanding of the complex regulatory network between transcription factors and histone modification at the beginning of life.
The first authors of this article include XU Ruimin, a doctoral student of Tongji University, Dr. LI Sen of the Second People's Hospital of Guangdong Province, Dr. WU Qiu and Dr. LI Yu of Tongji University, and Dr. JIANG Manxi, also of the Second People's Hospital of Guangdong Province. Professor GAO Shaorong, Professor OU Xianghong, Professor LIU Xiaoyu and Professor WANG Chenfei are the co-correspondents of this article. The research was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China, the Guangzhou Biological Island Laboratory and the Shanghai Science and Technology Commission.
Link to article: https://doi.org/10.1016/j.stem.2022.06.001