A methyl group is a common group in small molecule medicine, which may change its molecular conformation, improve its solubility, and enhance its metabolic stability and biological activity. This is known as the "methyl effect". Because of the "methyl effect", the development of methylation reaction and methylating reagents becomes an important goal for synthetic organic chemistry. Among molecules containing methyl, the 2,6-dimethyl-substituted aromatic is a common structure. Six of 200 small molecule medicine best sold in 2018 contain the active ingredients of this structure. It is common to introduce methyl to aromatic rings by transition metal-catalyzed coupling reaction and C-H bond methylation reaction. However, problems are reported in the existing reactions. For example, methylation reagents are highly toxic or are difficult to process. C-H bond methylation reaction depends on the presence of the controlled methyl, which brings about the ortho-methylation in most of the reactions
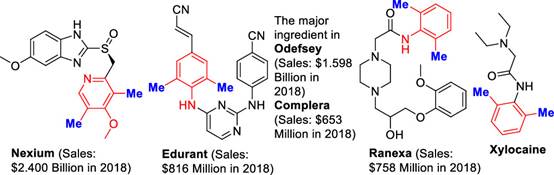
Fig.1 Drugs with an ortho, ortho-dimethylated arene moiety
Prof. ZHANG Yanghui's team from the School of Chemical Science and Engineering has been working on the organic reactions of carbon and carbon-cyclic palladium complex for years. For their special structures and unique reactivity, the complex can be used to develop novel and useful organic reactions. Prof. ZHANG’s team reported their research findings on “Pd-Catalyzed ipso, meta-Dimethylation of ortho-Substituted Iodoarenes via a Base-Controlled C–H Activation Cascade with Dimethyl Carbonate as the Methyl Source” in the Journal of the American Chemical Society.
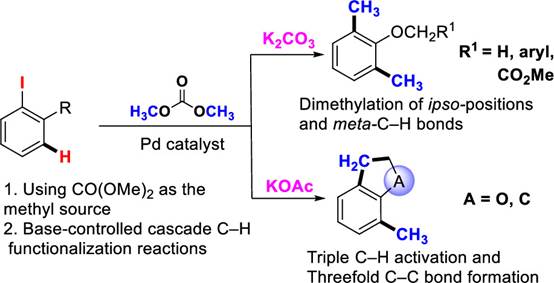
Fig. 2 Transition-metal-catalyzed C–H methylation
The reaction features the environment-friendly dimethyl carbonate as the methyl source, and achieves the methylation of 2-iodoanisole derivatives at the ipso position and meta position of iodine atom under the catalyzation by palladium. Dimethyl carbonate is an ideal methylation reagent as it is inexpensive, easy to use and does not produce hazardous chemical waste. The reaction uses dimethyl carbonate as methyl source in a coupling reaction as well as in a C-H bond methylation reaction for the first time. Its significance lies in the fact that inorganic bases can be used in the reaction to control the formation of the products, namely, the 2,6-dimethylanisole with potassium carbonate as a base; or the methyl-substituted 2,3-dihydrobenzofuran with potassium acetate as a base. The reactions involve multiple C(sp3)-H bond activation and the formation of multiple C-C bonds, especially the coupling of C(sp3)-H bonds, which is a novel cascade reaction. The 2,6-dimethylanisole and 2,3-dihydrobenzofuran formed in the reaction are not only important intermediates for organic synthesis, but also found in bioactive molecules and functional material molecules. The method developed in this study provides a simple and efficient way to obtain both types of compounds in a cascade reaction, starting with an easily prepared 2-iodoanisole substrate.
Prof. ZHANG Yanghui is the corresponding author of the paper while his doctoral student WU Zhuo the first author. The research is funded by the National Natural Science Foundation of China.
Link of the paper: https://pubs.acs.org/doi/10.1021/jacs.0c13057