Recently, a research paper entitled “Selective electrocatalytic reduction of oxygen (O2) to hydroxyl radicals via 3-electron pathway with FeCo alloy encapsulated carbon aerogel for fast and complete removal of pollutants” by Prof. ZHAO Guohua and Prof. ZHAO Yinghong’s team from the School of Chemical Science and Engineering (SCSE) was published in Angewandte Chemie International Edition, a leading international journal in the field of chemistry. Their research results provide an innovative strategy for rapid and efficient removal of environmental pollutants with the presence of O2 as a green oxidant and selective electrocatalytic reduction of O2 to hydroxyl radicals via a 3-electron pathway. The proposed strategy breaks through the classical Fenton reaction of using hydrogen peroxide as the oxidant, offering a green and efficient water treatment technology without using hydrogen peroxide and iron salt reagents.
Research on water environment and pollution control has long been centered on the development of new, green and efficient technologies for water pollution control, which has a direct bearing on the ecological safety of water resources and human health. The Fenton reaction, involving environmental-friendly advanced oxidation processes (AOPs), follows relatively simple operating principles and enables the efficient generation of hydroxyl radicals (∙OH), which are reactive oxygen species (ROS). The Fenton reaction possesses the advantages of strong oxidation and high mineralization, which has gained wide attention from researchers in the field of environment and water pollution control. However, the production of ∙OH in the traditional Fenton process is limited by the slow reaction rate of the Fe (II)/Fe (III) cycles and the narrow scope of pH application, which is a critical problem to be solved in practical water treatment.
From economic and environmental points of view, O2 is an ideal oxidant since it is abundant and cheap. However, O2 cannot be used directly for the degradation of organic pollutants because it is highly stable at normal temperatures and pressure levels. This is because O2 is a trilinear molecule that is forbidden to react with monoclinic organic pollutants and must be catalyzed and activated before it can be used as an oxidant. Therefore, a key frontier trend is to circumvent the drawbacks of the Fenton reaction using O2 as oxidant for green and efficient generation of OH. This is currently a research hotspot in the environmental field for upgrading APOs such as the Fenton process.
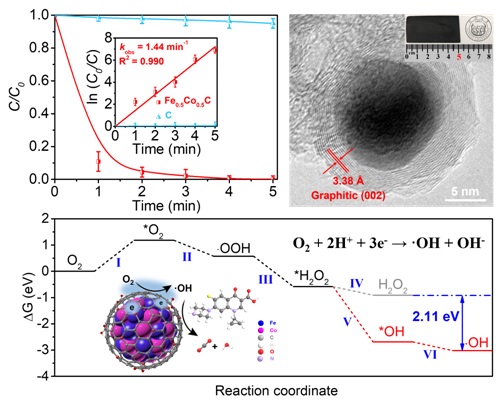
In their research work, Prof. ZHAO Guohua and Prof. ZHAO Hongying proposed an innovative strategy of designing FeCo alloy-encapsulated carbon aerogel (FeCoC) as a working electrode directly, indicating that the electrode has excellent electrochemical properties such as high specific surface area, space utilization, electrical conductivity and structural ability. The research results show that the active site of the electrocatalytic reduction of O2 is distributed on the surface of the graphite carbon shell with 2-electron electrocatalytic reduction of O2 to produce H2O2 first, followed by 1-electron electrocatalytic reduction of H2O2 toward OH. It is validated through an in-situ pressure test that shows the whole O2 reduction as an electrochemical process with the electron transfer number of 3.1. Completely different from the traditional Fenton reaction, the whole electrochemical process happens with the catalytic active site distributed on the surface of the graphite carbon shell, enabling the FeCo alloy in the FeCoC electrode to be perfectly protected by the outer graphite shell so as to allow no contact reaction with any external solution, nor any metal ion leaching or iron sludge generation. This process not only overcomes the rate control of slow valence cycling of Fe (II)/Fe (III) in the traditional Fenton reaction, but also maintains the efficient generation of ∙OH in the pH range of 3.0–11.0. and with excellent electrode cycling stability, the proposed strategy is applicable for the degradation of organic pollutants. Indeed, it enables pollutants to be degraded completely within five minutes, suggesting a satisfactory stability for practical applications. Theory calculations were applied to fully investigate the molecular mechanism of selective production of ∙OH, X-ray absorption near-edge structure spectroscopy, near-edge X-ray absorption fine structure spectroscopy, electron paramagnetic resonance spectroscopy, in situ pressure method, linear scanning voltammetry, and density flooding. The proposed theoretical system of interfacial electron modulation selective electrocatalytic reduction of O2 to generate ∙OH in the research work provides an important theoretical basis for both the research and application of new methods for green and efficient oxidative degradation of toxic and hazardous organic pollutants, thus contributing to expanding waste water treatment technologies.
Prof. ZHAO Guohua and Prof. ZHAO Hongying are the corresponding authors of the paper, and XIAO Fan, a master student from the SCSE, is the first author of the paper. The authors acknowledge funding from the National Natural Science Foundation of China.
Paper source:https://doi.org/10.1002/anie.202101804