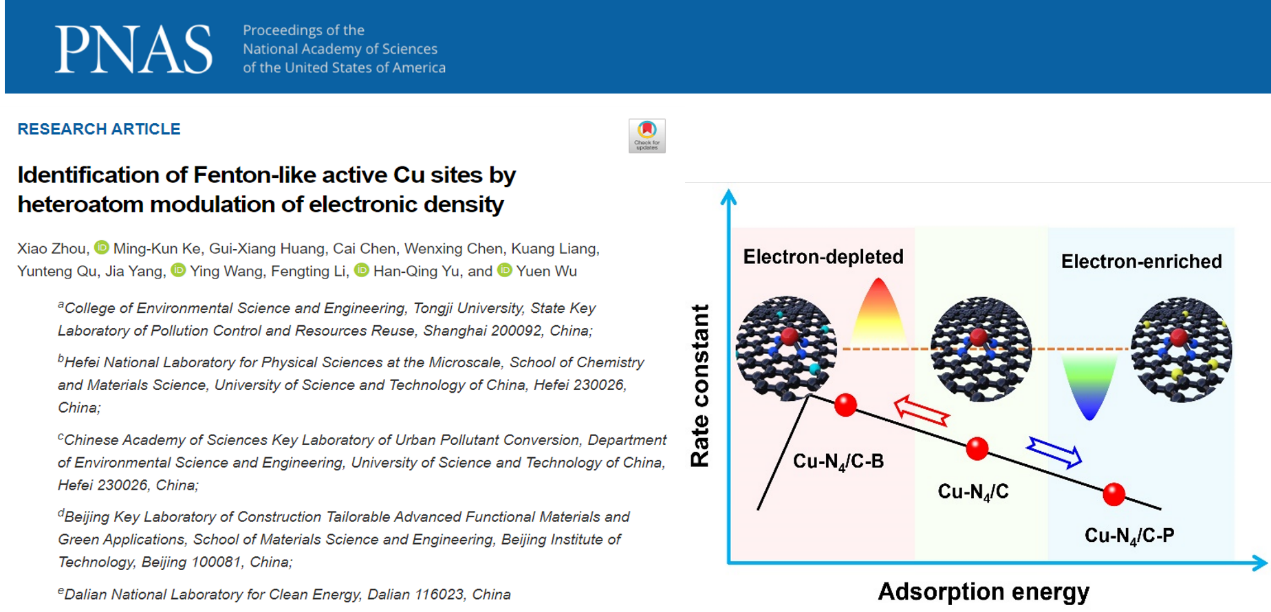
Recently, Professor WANG Ying's team from the College of Environmental Science and Engineering and a research team from the University of Science and Technology of China published a paper entitled "Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density" in the international journal Proceedings of the National Academy of Sciences (PNAS). By introducing electron-deficient boron (B) or electron-rich phosphorus (P) into the carbon substrate, the electron density of Cu centers was systematically modulated and its effect on the reaction kinetics of activated PMS degradation of bisphenol A (BPA) was examined. It turned out that the Cu-N4/C-B has the best catalytic oxidation capacity, which is superior to the vast majority of non-homogeneous Fenton type catalysts. This research provides insights into the electronic structure regulation of single-atom metal centers and the structure-activity relationship at the atomic level.
The paper published in PNAS by Prof. WANG's team in collaboration with a research team from the University of Science and Technology of China.
Organic pollutants in water pose a serious threat to human health and ecological environment. In particular, persistent organic pollutants in wastewater are environmentally highly toxic and hard to degrade, which makes it difficult or impossible for traditional physicochemical and biochemical treatment to meet the technical and economic requirements for the purification and treatment of such pollutants. Fenton/Fenton-like reactions treat various organic pollutants in water by generating strong oxidizing reactive oxygen species. Among them, peroxymonosulfate (PMS) has caused much attention in recent years for its advantages of being easy for storage and transportation and its advanced oxidation technology PH being widely applicable. However, the slow kinetics of PMS in the activation process restricts its development. Homogeneous transition metal ion system has a high PMS activation capacity, but it is poor in recycling and easy to generate sludge. In contrast, common materials such as non-homogeneous transition metal oxides and loaded nanoparticles have low metal atom utilization and slow reaction kinetics.
Single-atom catalysts with unique electronic structure and maximum atom utilization efficiency provide a new solution to the above problems. In addition, the electronic structure of single-atom active sites has an effect on the kinetics of catalytic reactions. How to regulate the electronic structure of single-atom catalysts is essential to further enhance the reaction kinetics of PMS. Therefore, in this study, a series of heteroatomic (B/P)-modified Cu single-atom catalysts (Cu SAs) were constructed with an intent to enhance the PMS reaction kinetics for organic pollutant degradation, and the Cu single-atom electronic structure and Fenton-like catalytic activity were revealed. With integration of synchrotron X-ray absorption spectroscopy with theoretical calculation, the electron density variation pattern of Cu sites in the monatomic catalysts was explored, and their effects on the reaction kinetics of BPA degradation by activated PMS examined, and the mechanism of the role of Cu monatomic catalysts in the process of activated PMS elucidated.
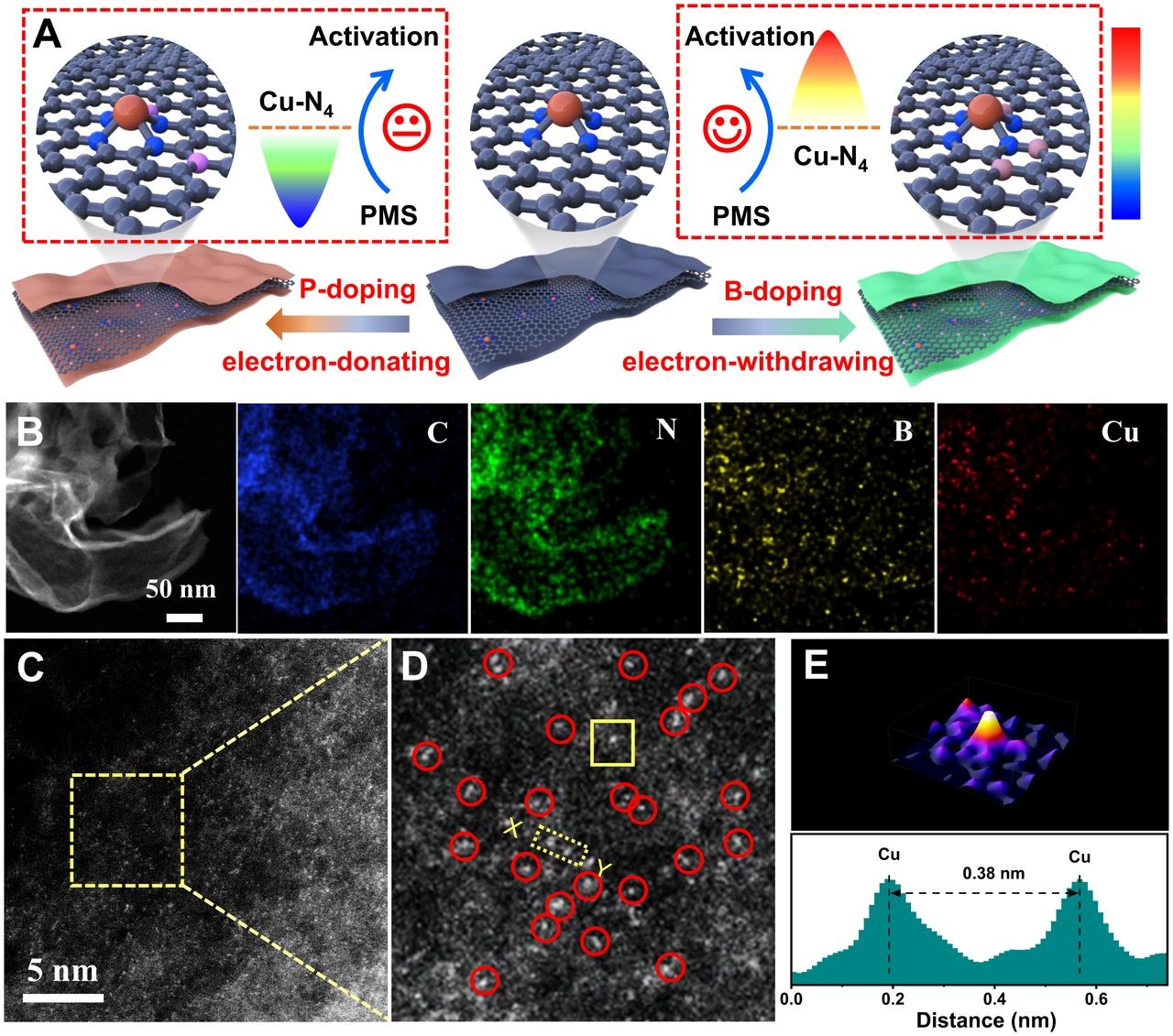
(A) Schematic of the preparation strategy for Cu-N4/C-B and Cu-N4/C-P. The color bar indicates the electronic density of Cu-N4 site, electro-rich (blue) and electro-poor (red).
(B) HAADF-STEM image and the corresponding EDS mapping images of Cu-N4/C-B.
(C) AC HAADF-STEM image and
(D) enlarged intensity image of Cu-N4/C-B.
(E) Atom-overlapping Gaussian-function-fitting mapping of the square from D, intensity profile along X–Y in D.
In this research, the electron density of Cu centers was systematically adjusted by introducing electron-deficient B elements or electron-rich P elements into the carbon substrate, and their effects on the reaction kinetics of activated PMS for the degradation of bisphenol A were investigated. Among them, the electron-deficient Cu-N4/C-B has the best catalytic oxidation, better than the vast majority of non-homogeneous Fenton type catalysts. In contrast, the electron-rich Cu-N4/C-P catalyst leads to a decrease in activating PMS. Both experimental results and theoretical calculations show that the long-range action of B atoms can reduce the electron density of the Cu active site, shift the d-band center downward, and optimize the activation of PMS adsorption. Fine tuning of the electronic structure of Cu sites at the atomic level optimizes the activation PMS reaction kinetics and provides theoretical guidance and technical support for the design of advanced Fenton-like catalyst materials.
The corresponding authors of the paper are Prof. WANG Ying from the College of Environmental Science and Engineering, Tongji University, Prof. YU Hanqing and WU Yuen from the University of Science and Technology of China, the first author is ZHOU Xiao, a postdoctoral researcher from the College of Environmental Science and Engineering, Tongji University, and the co-author is Ke Mingkun, a PhD student from the University of Science and Technology of China. This project was supported by the National Natural Science Foundation of China.
.
Link of paper:https://www.pnas.org/content/119/8/e2119492119
https://news.tongji.edu.cn/info/1002/80062.htm