Blocking microtubules to inhibit cell proliferation and
limiting energy supply to promote cell apoptosis
Drug-resistant tumors can be effectively treated by blocking microtubules to inhibit cell proliferation, and limiting energy supply to promote apoptosis through the self-assembly of intracellular peptides. This "non drug therapy" provides a new approach for the treatment of clinically advanced and drug-resistant tumors. On April 1, research results by DU Jianzhong and FAN Zhen’s team from the School of Materials Science and Engineering of Tongji University were published on the supplementary cover of Journal of the American Chemical Society under the title of Intracellular Self-Assembly of Peptides to Induce Apoptosis against Drug-Resistant Melanoma.
The paper published on the supplementary cover of Journal of the American Chemical Society
Chemotherapy, as one of the main ways to treat tumors, is likely to induce tumor drug resistance, resulting in "no drug to treat". Therefore, the research on "non drug therapy" for drug-resistant tumors has important clinical value and social significance.
Microtubule, an important cytoskeleton composed of tubulin, participates in the formation of centrosome in the late stage of mitotic DNA synthesis (G2 phase). During the tumor cell division (M phase), the centrebody will guide the cell division, which means tumor expansion. Therefore, inhibiting the formation of microtubules may inhibit tumor expansion. In addition, as the main place of cell energy metabolism, mitochondria not only affect cell division, but also induce apoptosis during dysfunction, thus inhibiting drug-resistant tumors.
At present, chemotherapeutic drugs are mainly used to inhibit the formation of microtubules and interfere with mitochondrial function. However, it is difficult for patients to bear the high systemic toxicity caused by repeated and long-term use of chemotherapeutic drugs. Therefore, a challenging question arises: how to inhibit the formation of microtubules in tumor cells and lead to mitochondrial dysfunction through "non drug therapy", and treatment of drug-resistant tumors?
To answer this question, the research team asked whether a non-toxic short peptide can be synthesized to self-assemble into nanoparticles in cells, regulate the cell cycle (G2 / M) by interfering with tubulin polymerization and interfere with mitochondrial function, so as to treat drug-resistant tumors.
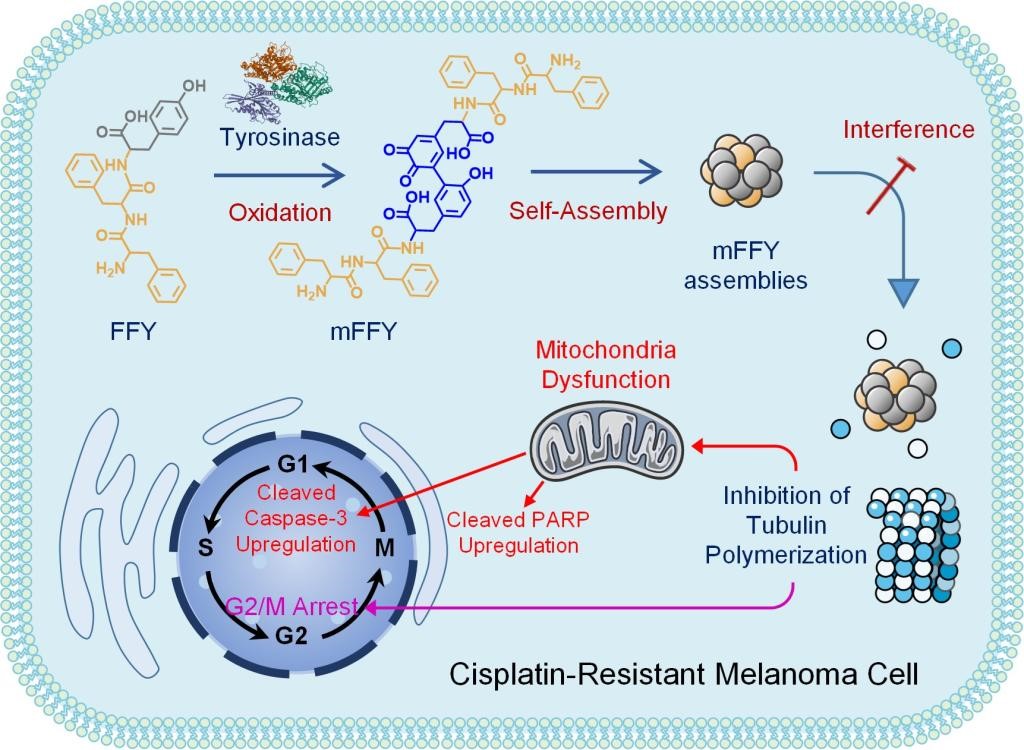
Figure 1: "non drug therapy": blocking microtubules to inhibit proliferation, and limiting energy supply to promote apoptosis. Polypeptide intracellular enzymatic self-assembly induces G2 / M phase arrest and mitochondrial dysfunction to reverse the drug resistance of melanoma.
The research team proposed the "non drug therapy" of "blocking microtubules to inhibit proliferation and limiting energy supply to promote apoptosis", and carried out their research with drug-resistant melanoma as a tumor model. The researchers introduced tripeptide (FFY) into drug-resistant melanoma cells, which formed mFFY nanoparticles through enzymatic self-assembly in the cells. It was found that after CO incubation of mFFY nanoparticles with tubulin, the nanoparticles effectively inhibited tubulin polymerization.
As shown in Figure 2, following the "non drug therapy" treatment, most tumor cells stagnate in the late stage of DNA synthesis or division interval (i.e. G2 / M block) due to the inability of tubulin to polymerize into microtubules, thus inhibiting the proliferation of tumor cells. At the same time, mitochondrial dysfunction also induced the overexpression of apoptosis factors, such as cleaved caspase 3 (3.1 times higher than the control group) and cleaved PARP (6.3 times higher), which further promoted the apoptosis of drug-resistant tumor cells and finally realized the reversal of tumor drug resistance.

Figure 2: mechanism of enzyme induced ffy reversing drug resistance in melanoma
In vivo experiments have also proved that "non drug therapy" can effectively reverse tumor drug resistance. Animal experiments showed that after injection of FFY around melanoma, a high level of drug-resistant tumor inhibition was observed, that is, the tumor volume decreased by 87.4% compared with the control group after two treatments (as shown in Figure 3). At the same time, compared with the chemotherapy drug treatment group, "non drug therapy" can effectively reduce or avoid systemic toxicity.
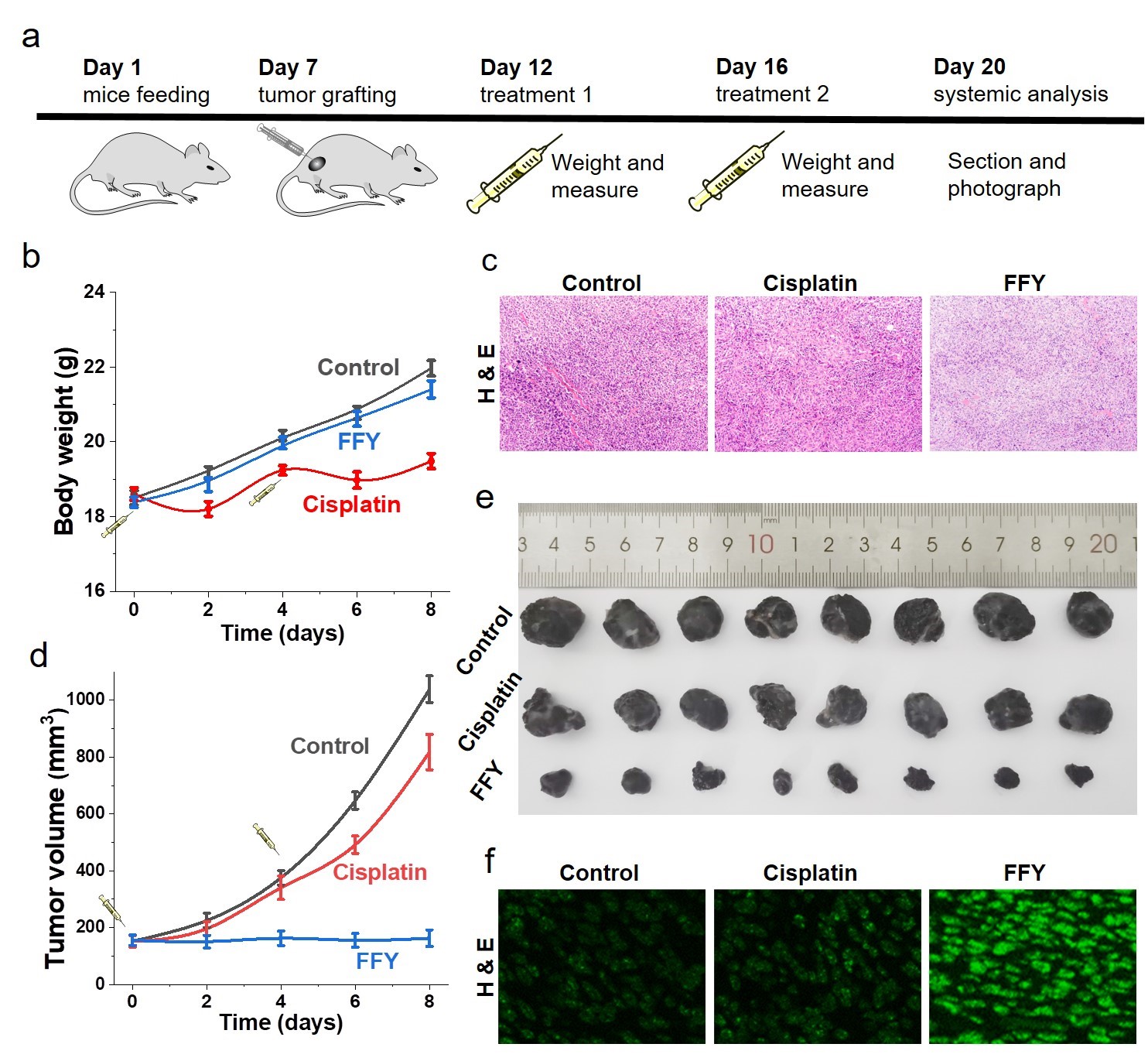
Figure 3: Inhibitory effect of "non drug therapy" on drug-resistant melanoma
SUN min, a doctoral student in the Department of polymer materials, School of Materials Science and Engineering, Tongji University, is the first author of the paper, and Professor DU Jianzhong and researcher FAN Zhen are the corresponding authors. The project has been supported by the National Natural Science Foundation of China and the National Science Foundation for Distinguished Young Scholars.
Link to paper: https://pubs.acs.org/doi/abs/10.1021/jacs.2c00697
Source: https://news.tongji.edu.cn/info/1003/80459.htm