Professor WANG Zhiwei's team from the School of Environmental Science and Engineering (SESE) of Tongji University, in collaboration with Professor LIN Shihong from Vanderbilt University and Professor TANG chuyang from the University of Hong Kong, developed a new polyamide RO membrane with ultra-high selectivity for efficient removal of boron and NDMA in water (> 90%). They succeeded in controlling the self-assembly heat and mass transfer of nano sheets at the free interface of oil and water in tackling the problem of low removal rate of boron and dimethylnitrosamine (NDMA) traditional reverse osmosis (RO) membrane in water reuse and seawater desalination. Their research results were recently published online in Science Advances, a journal of the Science family, entitled "Metal-organic framework enables ultraselective polyamide membrane for desalination and water reuse".

Paper by WANG Zhiwei's team published in Science Advances
RO membrane separation is an important technology in seawater desalination, sewage/wastewater advanced treatment and water reuse. However, although most of the existing commercial polyamide RO membrane can intercept salt in water by over 99.0%, its removal effect is still limited in some highly toxic small molecular pollutants (such as boron in seawater and disinfection by-product NDMA). In this research, an interface polymerization (MARIP) approach based on precise regulation of nanosheets self-assembly was proposed by introducing amphiphilic CuBDC metal organic framework nanosheets in the free interface of water/n-hexane in situ. Polyamide RO membrane with ultra-high selectivity was produced, which greatly improved the removal rate of high toxic small molecular pollutants boron and NDMA by RO membrane.
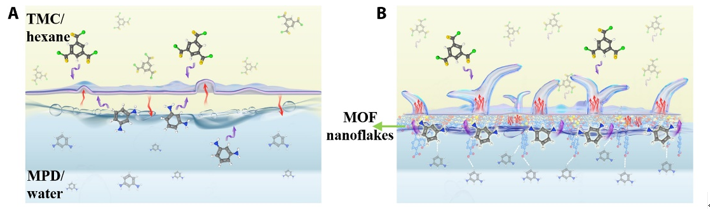
FIG. 1. Fabrication of ultraselective PA membrane using MARIP. (A) Graphical illustration of the IP process at a free interface [free interface polymerization (FIP)]. (B) Graphical illustration of MARIP, where the amphiphilic MOF nanoflakes conserved heat at the interface and accelerated MPD transport across the interface to form a crumbled PA active layer with a smaller intrinsic thickness and a higher degree of cross-linking
It was found that the water permeability of RO membrane prepared via MARIP was significantly improved, compared with the control group (FIP membrane). In addition, the rejection rate of NaCl was increased synchronously, from 95.4% (FIP) to 99.5% (MARIP-0.1, the nano tablets introduced 0.1 wt%). Compared with the RO membrane reported in the literature, MARIP-0.1 membrane has very high water/NaCl selectivity. Meanwhile, MARIP-0.1 membrane has high rejection rates for boron and NDMA (90.1% and 90.3% respectively), and has high water/boron selectivity, which is higher than that of RO membrane reported in the literature.
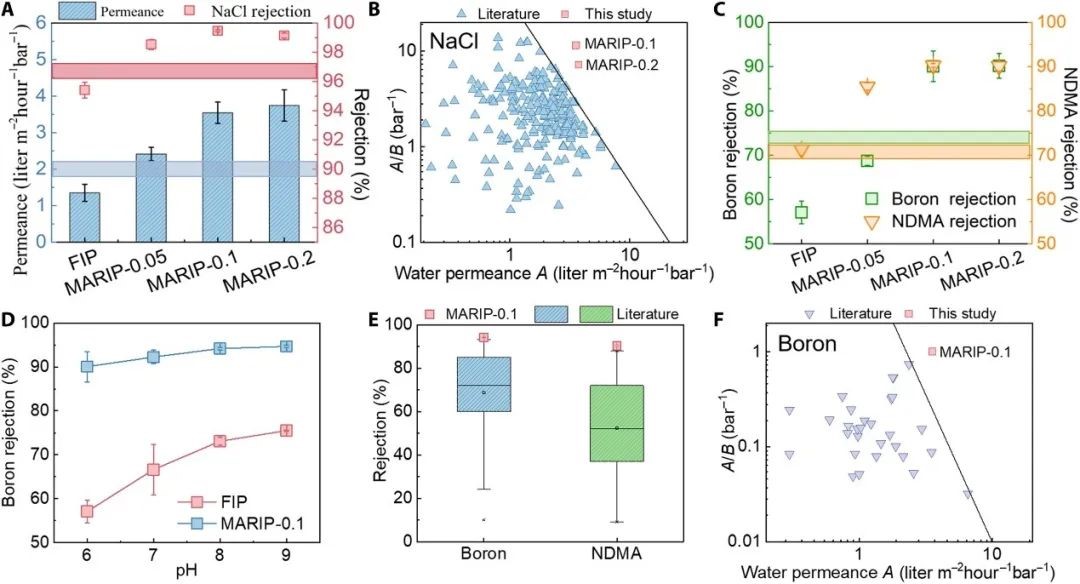
FIG. 3. Membrane separation performance.
(A) Pure water permeance and NaCl rejection [NaCl (2000 mg/liter), 16 bar, 24°C] (n = 6). The blue and red horizontal bars represent the water permeance and NaCl rejection of conventional TFC-PA membrane, respectively (the thickness represents SD). (B) Separation performance in terms of water permeance and water/NaCl selectivity of MARIP-0.1 and MARIP-0.2 membranes (red) as compared with literature data (blue). (C) Rejection of boron (5 mg liter−1, pH 6.0, 16 bar, 24°C) and NDMA (890 μg liter−1, 16 bar, 24°C) (n = 6). The green and orange horizontal bars represent the rejection of boron and NDMA by conventional TFC-PA membranes, respectively. (D) Rejection of boron (5 mg liter−1, 16 bar, 24°C) by FIP and MARIP-0.1 membranes at different pH. (E) Comparison of the boron (pH 8) and NDMA rejection performance of MARIP-0.1 with data reported in literature. (F) Separation performance in terms of water permeance and water/boron selectivity of MARIP-0.1 membrane (red) as compared with literature data (purple).
A closer study at the structure of the membrane showed that when there was no nano sheet to regulate the free interface polymerization, the surface of the FIP membrane was fairly flat (the inherent thickness of polyamide layer was ~ 16 nm). With the introduction of nanosheets into the water/ il interface to regulate the formation process of polyamide, typical "peak and valley" structures appear on the membrane surface, especially for MARIP-0.1 and MARIP-0.2 membranes, large leafy structures appear most of the membrane surface. It was also found that with the introduction of nano sheets, the inherent thickness of polyamide layer was reduced to ~ 5 nm, the specific surface area and cavity proportion of the membrane were significantly increased, and the crosslinking degree of the membrane also significantly improved.
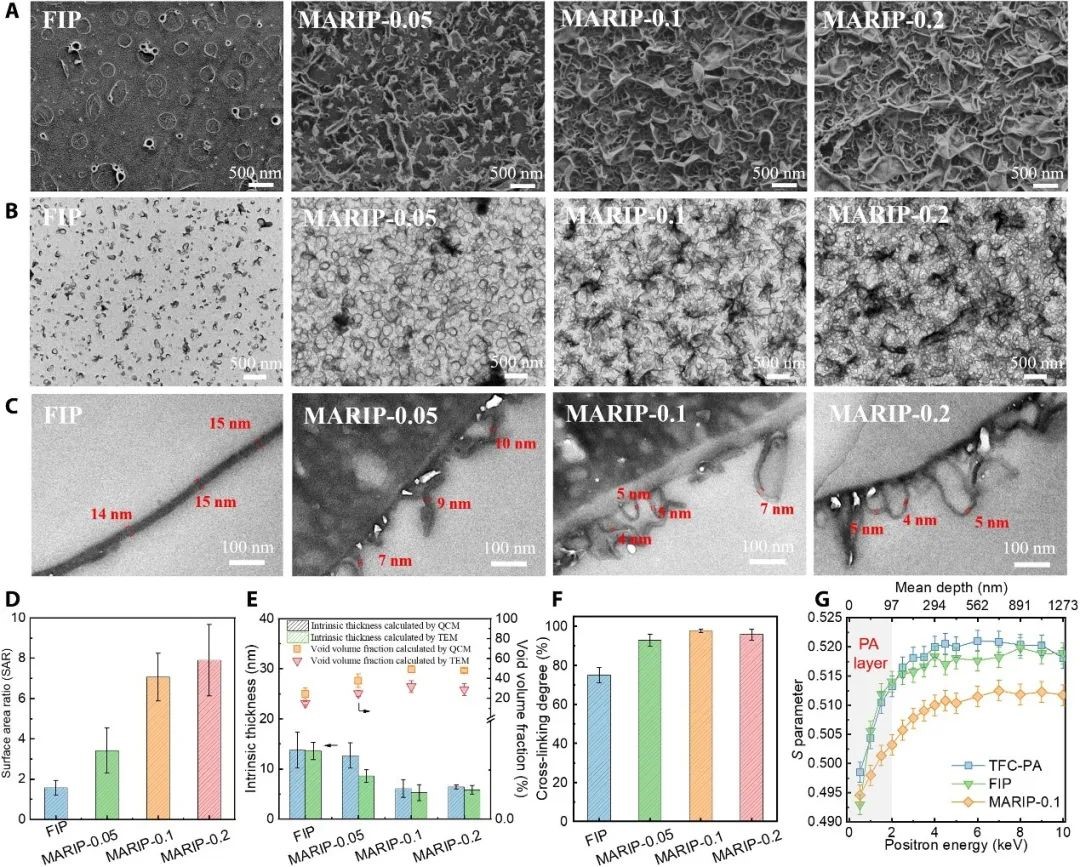
FIG. 2. Morphologies and properties of FIP, MARIP-0.05, MARIP-0.1, and MARIP-0.2 membranes.
(A) SEM micrographs of the top surfaces. (B) Top-down TEM micrographs. (C) Cross-sectional TEM micrographs. (D) Surface area ratio (SAR) from analyzing cross-sectional TEM images (n = 10). (E) Intrinsic thickness and void volume fraction measured using quartz crystal microbalance (QCM; n = 3) and TEM (n = 10). (F) Degree of cross-linking. (G) Evolution of S parameters for the PA nanomembranes obtained from conventional IP with support layer (TFC-PA), FIP, and MARIP (0.1% MOF).
The study further revealed the mechanism of how nano sheets improve the permeability/selectivity of MARIP RO membranes: 1) the addition of amphiphilic nano sheet helps to reduce the interfacial tension of the system, and the nano sheet has preconcentration effect on the aqueous monomer m-phenylenediamine (MPD), which is conducive to the cross-Interface diffusion of MPD and promotes the interfacial polymerization; 2) when the nano sheets are introduction into the free interface, the exothermic heat of interfacial polymerization accumulates due to the thermal insulation effect of nano sheets, which promotes the interfacial polymerization reaction, increases the crosslinking degree of the membrane and improves the selectivity of the membrane; 3) the interface exothermic accumulation promotes the dissolution of nano bubbles in the solution. The nano bubbles play the role of template, form nano cavities in the polyamide layer, improve the specific surface area of the membrane, and improve the water permeability of the matrix membrane. The research provides an insight into improving the efficiency of RO membrane technology in seawater desalination, sewage/wastewater advanced treatment and water reuse.
Dr. WEN Yue and DAI Ruobin, an assistant professor, both from SESE of our University are the first and second authors of the paper. Other authors are LI Xuesong, an associate researcher of SESE, Dr. ZHANG Xingran, Professor WU Zhichao, CAO Xingzhong, researcher from the Institute of High Energy Physics of the Chinese Academy of Sciences. Professor LIN Shihong of Vanderbilt University, Professor TANG chuyang of the University of Hong Kong and Professor WANG Zhiwei of Tongji University are the corresponding authors. The research was supported by the National Natural Science Foundation of China Distinguished Young Scholars project.
Link to paper:https://www.science.org/doi/10.1126/sciadv.abm4149
Source: https://news.tongji.edu.cn/info/1003/80469.htm