On July 5, the United Nations World Intellectual Property Organization (WIPO) and Tongji University jointly invited Professor William Fisher from Harvard University of the United States, Professor Stefan Wagner from the European School of Management and Technology (ESMT), Berlin, Germany, and Associate Professor Sven Bostyn from the University of Copenhagen, Denmark, to give three academic reports on specific issues. More than 200 people, including teachers and students of Shanghai International College of Intellectual Property (SICIP) of Tongji University and embers of WIPO International Network for Intellectual Property Education (WINIPe) attended the online lecture. The lecture also attracted enthusiastic participation and in-depth discussion from domestic colleges and industrial circles.
In his presentation on The Global Health Crisis: A Bird’s Eye View of Problems and Potential Solutions, Professor Fisher pointed out that both infectious and parasitic diseases have become the top two causes of death in the world and the main threat to life expectancy in low-income countries. This is on the basis of global mortality and incidence rate data of various diseases in 2019. Based on his historical experience in the prevention and control of infectious diseases in the United States, he pointed out that the public health system, vaccines and drugs are the key factors to reduce the mortality rate of infectious diseases. It follows that accessibility to drugs and vaccines, counterfeit drugs and slower new drug innovation have all become core issues that limit the ability of low-income countries to prevent and control infectious diseases. Professor Fisher made suggestions on how to reduce the imbalance of medical resources between low-income countries and high-income countries and lessen unnecessary disease deaths in low-income countries. In the Q & A Session after the lecture, Professor LI Mingde of the Chinese Academy of Social Sciences pointed out that drug innovation was crucial to solving the health crisis in combination with China's experience in solving the food problem through agricultural scientific and technological innovation. Professor SONG Xiaoting from the National Academy of Sciences introduced China's experience in balancing drug patent protection and public health interests from the perspectives of the expansion of patentable objects of biomedicine, compulsory licensing and open licensing. The scholars at the meeting had an intense discussion on such issues as the right to life, compulsory and open licensing, patent monopoly, and vaccine patents.
WIPO - Tongji annual academic seminar online
Based on the supply and demand structure of the pharmaceutical market, Associate Professor Bostyn held that the pricing and compensation rules would greatly affect the competition between drugs in his presentation with the title of "Drug accessibility and the challenge of monopoly". Pharmaceutical enterprises need to overcome multiple obstacles to introduce new drugs to the market, and there are high risks and uncertainties. Associate Professor Bostyn further analyzed the Push and Pull incentives of the pharmaceutical industry. He analyzed in detail the conflict between drug patent rights and public health from the three fields of reuse, rare use & multiple indications of drugs, medical product accessibility and epidemic control. He put forward suggestions on how to build a drug patent system that can consider both innovation incentives and public health interests. In the Q & A Session that followed the lecture, Professor Joseph Straus, former director of the Max Planck Institute for Innovation and Competition in Germany, reviewed the solutions proposed by Bostyn, and pointed out that the corresponding solutions should be based on empirical data. Prior to having corresponding data support and justification, legislators should be cautious. From the perspective of comparative law, Associate Professor PENG Zhe of the School of Law of Shandong University introduced the right of intervention, similar to the compulsory license system in American law. He pointed out that although there are March-in Rights under the Bayh-Dole Act, there has been no cases of compulsory licenses yet in the United States according to the report of the US Congress. The scholars at the meeting had a wide-ranging discussion about the Bayh-Dole Act, compulsory licenses, drug patent link, drug patent term compensation, drug data exclusive period among other issues.
Online discussion among scholars
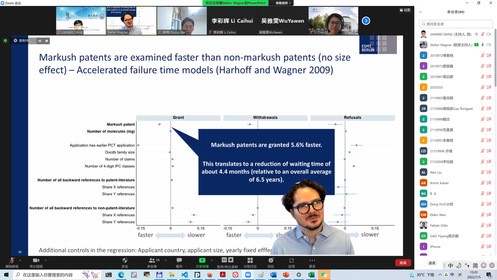
Professor Stefan Wagner of the European Institute of Management and Technology reported on his newly published paper "Mapping Markush" on research policy, discussing the impact of Markush patents on patent examination and pharmaceutical innovation. Markush structure is a special chemical molecular make-up. The patent claims written in Markush structure can protect a class of chemicals with similar structures. There are many disputes in the academic and industrial circles on the examination burden and patent barriers of a Markush patent, but these differences have not been tested by empirical research for a long time. Based on a large number of medical patent data, Professor Wagner proved empirically for the first time that, unlike the general cognition, a Markush patent does not lead to the extension of the patent examination cycle, but rather is easier to form patent barriers and reduce the licensing rate of subsequent invention patent applications. Intellectual property directors and drug R & D project directors from well-known pharmaceutical enterprises had an intense discussion with researchers in the academic circles around Professor Wagner's speech. Experts in the industry generally believe that the protection of Markush patents in China is reasonable in balancing the interests of innovators and the public. Although the Markush patent forms a barrier to micro pharmaceutical innovation by fine-tuning the molecular structure, it can encourage pharmaceutical enterprises to explore more ways of drug R & D technology, and objectively increase the types of new drugs available to the public. HUO dong, Associate Professor of Harbin Institute of Technology (Shenzhen), CHEN Li, Assistant Professor of Gothenburg University in Sweden, Professor REN Shengce and Assistant Professor LIU Xia, both from China’s National Academy of Sciences, held in-depth exchanges with Professor Wagner and industry experts on issues such as the license of Markush patents, the decision-making of enterprise in drug R & D and patent strategy, and the review cycle of Markush patents.
Professor Stefan Wagner delivering a keynote speech