For a long time, due to the limitations of ethics and technology, research on embryo development and related diseases at implantation stage has been slow. The stem cell-based embryo in vitro simulation technology provides a good platform for researchers, especially concerning the construction of blastocyst like-embryos which to a large extent allows researchers to operate and observe the continuous process covering pre-implantation to post implantation.
In 2019, the "expanded pluripotent stem cell blastoid (EPS-blastoids)" structure was constructed by the Belmonte laboratory using mice expanded pluripotent stem cells (EPS). This is a kind of blastoid structure that includes the complete primitive three lineages of trophoblast (TE), primordial endoderm (PrE) and Epiderm (EPI)[5]. Unfortunately, EPS-blastoids have serious post implantation development defects and cannot form functional post implantation embryos, which greatly limits its further application.
On July 15, GAO Shaorong's research group of Tongji University published an online research paper entitled "Bilineage Embryo-like structure from EPS cells can produce live mice with tetraploid trophectoderm" in Protein & Cell. This study elucidated the reasons and mechanisms of post implantation developmental defects in EPS-blastoids, and further attempted to construct functional blastocysts.

Research results by GAO’s team published in Protein & Cell online
The researchers first tested the lineage specific genes of EPS-blastoids. They found that the expression of the trophoblast cell marker CDX2 in the TE-like structure of EPS-blastoids was much lower than that in the TE cells of normal blastocysts and that the TE-like structure generally expressed the specific genes GATA6, SOX17 and PDGFRα of the PrPlineage. Subsequently, the researchers verified the post implantation development ability of EPS-blastoids using in-vivo transplantation experiments and post implantation embryo invitro culture (IVC). The results showed that EPS-blastoids, like the embryoid bodies (EBs) produced by embryonic stem cells (ES), could induce decidual reaction in the in-vivo transplantation experiment, but that there was no normal post implantation structure in the decidua. However, in vitro experiments, most of the post implantation embryonic-like structures formed by EPS-blastoids contain EPI of NANOG+ and visceral endoderm (VE) of SOX17+, but lack TFAP2C+ embryonic ectoderm (ExE) lineage cells. The above experiments prove that TE-like structure in EPS-blastoids is not a typical TE-lineage cell.
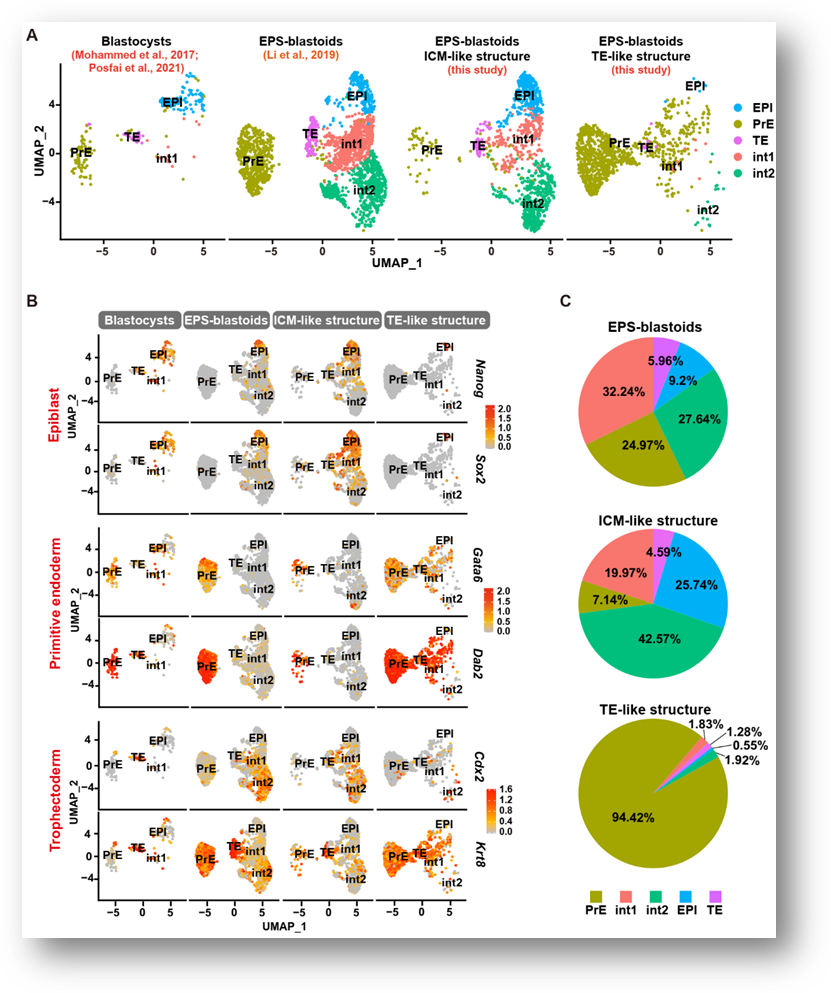
Figure 1. Single cell data analysis of TE-like and ICM-like structures in EPS-blastoids
In order to explore the reason why EPS-blastoids lack TE-lineage cells, the researchers performed single-cell sequencing on the TE-like structure and the intraclass cell mass-like (ICM-like) structure of EPS-blastoids. The results showed that TE-like structure was basically composed of PrE-lineage cells (94.42%), and a small number of TE cells (4.59%) were derived from ICM-like structure (Fig. 1). When the researchers studied the expanded pluripotency of EPS cells, they found that PrE like cells of GATA6 / SOX17 / PDGFRα +OCT4+ existed in EPS cells. Treatment with PrE- differentiation can significantly improve the efficiency of EPS and even ES cells to form EPS-blastoids (Fig. 2). On the contrary, if the differentiation of EPS cells in the direction of PrE is prevented by reducing GATA6, the formation of EPS-blastoids will not be possible. The above experiments confirmed that the tendency of EPS cells to differentiate into PrE-lineage cells is an important reason for the formation of EPS-blastoids.
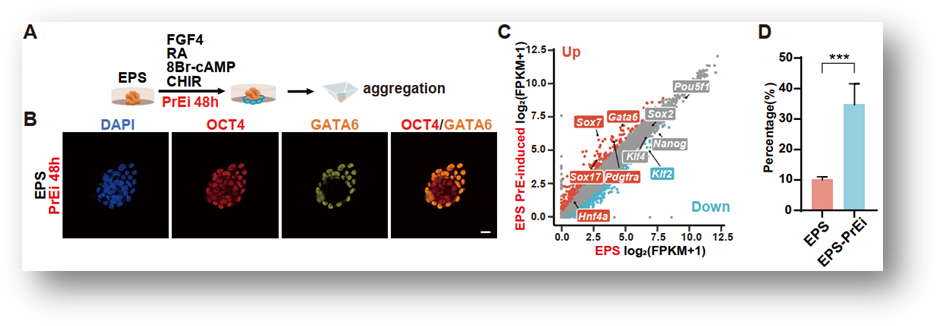
Figure 2. Induced differentiation in the direction of PrE promoting the formation of EPS-blastoids
in EPS cells
Functional blastocysts are composed of three cell lineages: trophoderm (TE), Epiderm (EPI) and primordial endoderm (PrE). The researchers found that although EPS-blastoids could not produce enough TE lineage cells, they could produce bilineage embryo-like structures (BLES) containing PrE and EPI during their formation. Because the lack of PrE is also a major obstacle in the construction of blastocyst-like embryos, researchers tried to use this BLES structure to construct functional recombinant embryos. The researchers injected BLES into the cavity of the expanded tetraploid blastocyst to recombine TE cells of tetraploid embryos with BLES. This reconstituted embryo successfully obtained normal pregnancy after transplantation, in which BLES could develop into fetus and yolk sac, and further produced fertile mice.
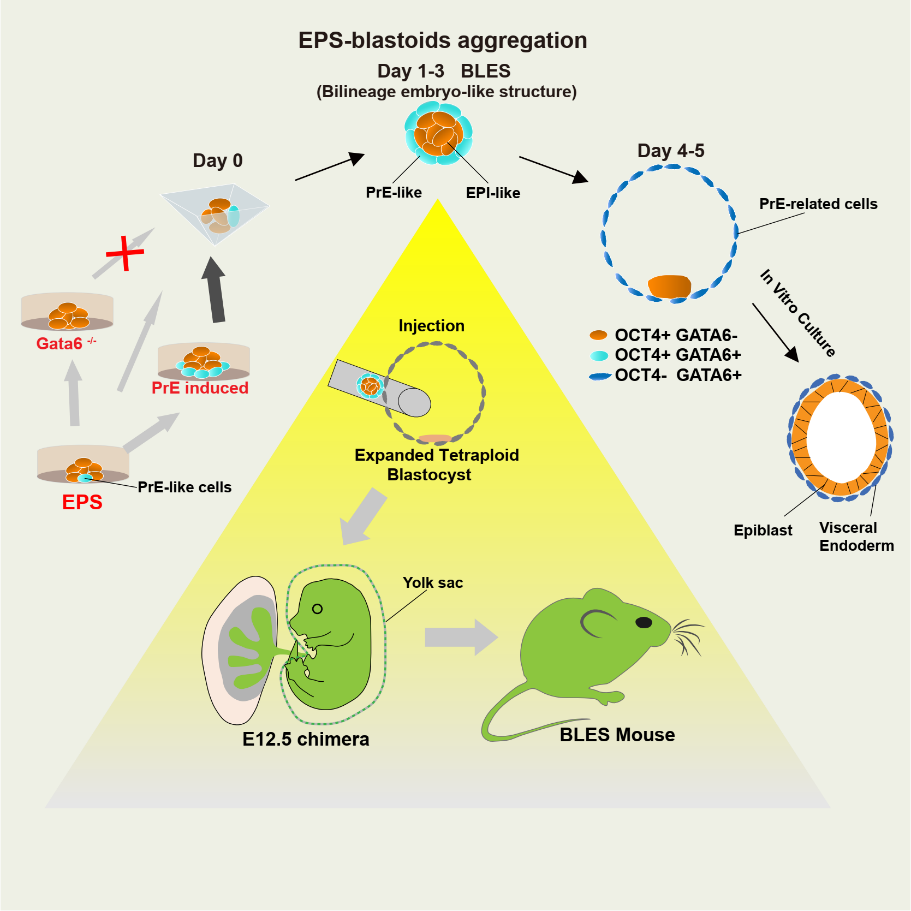
Figure 3. Although EPS-blastoids cannot produce enough TE lineage cells, they will produce BLES containing PrE and EPI during their formation
In conclusion, the researchers found that EPS-blasteids could not achieve post implantation development due to the lack of real TE-lineage cells, and its TE-like structure was composed of PrE-lineage cells. This study further used the test reconstitution of BLES containing EPI and PrE-lineage cells and tetraploid embryos to achieve the generation of fertile mice. This study provides a theoretical and experimental basis for the establishment of functional blastocyst-like embryos.
Dr. LIU Kuisheng, Dr. XU Xiaocui and Dr. BAI Dandan, from Professor GAO Shaorong's laboratory, are the co-first authors of the paper. Professor GAO Shaorong, researcher LIU Wenqiang and Professor WANG Yixuan are the co-authors of the paper. The research was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China and the Shanghai Science and Technology Commission.
Paper link:http://doi.org/10.1093/procel/pwac029