Cryptococcosis refers to an acute, sub-acute or chronic pulmonary fungal disease caused by Cryptococcus neoformans (C neoformans) and its variants. It is mainly caused by inhaling through the respiratory tract, from where it can be transmitted to the brain by the central system and seriously endanger the lives of immunocompromised patients. Clinical and experimental data indicate that CD4+T-cell-mediated immunity (CMI) is essential to control cryptococcal infection. However, an important contributor of cryptococcal infection is systemic immunosuppression which is characterized by poor CMI and a pro-inflammatory response. It follows that there is an urgent need to develop new immunotherapeutic strategies to enhance CD4+T cell responses against cryptococcal infection.
On July 14, Professor JIA Xinming of the Tenth People's Hospital, affiliated to Tongji University, and Professor QU Jieming of Ruijin Hospital, affiliated to the Medical College of Shanghai Jiao Tong University, published jointly in Nature Communications a research article entitled "Inhibition of myeloid-derived suppressor cell Arginase-1 production enhances T-cell-based immunotherapy against Cryptococcus neoformans infection". This study revealed that glucuronoxylomannan (GXM), the main capsular polysaccharide component of C neoformans, inhibits the clearance of C neoformans by inducing the accumulation and function of granulocyte-like myeloid, derived from immunosuppressive cells (PMN-MDSCs) in the body. This aggravates the infection. Therefore, identifying the recognition receptors and downstream pathways of GXM, provides a new potential immunotherapeutic target and strategy for fighting Cryptococcus infection.

The article published in Nature Communications
Myeloid derived suppressor cells (MDSCs) are a group of heterogeneous cells derived from myeloid progenitor cells and immature myeloid cells, which were defined in 2007. They have significant immunosuppressive effects. According to karyotype characteristics and surface markers, they are divided into two subtypes: monocyte-like M-MDSCs and granulocyte-like PMN-MDSCs. The researchers first found that PMN-MDSCs increased significantly in the mice trials of cryptococcal pulmonary infection, clinical cryptococcal pneumonia and cryptococcal meningitis. To further clarify the function of PMN-MDSCs in cryptococcal infection, the researchers successfully cleared PMN-MDSCs in mice with anti-Ly6G and anti-DR5 antibodies respectively. The results showed that this could significantly increase the survival rate of mice after they were exposed to cryptococcal infection and reduce the amount of bacteria in the lungs (Fig. 1). The research indicates that C neoformans infection can induce the recruitment of MDSCs, especially PMN-MDSCs, and aggravate the infection process. Neutralizing such immunosuppressive cells can reduce the impact of C neoformans infection.
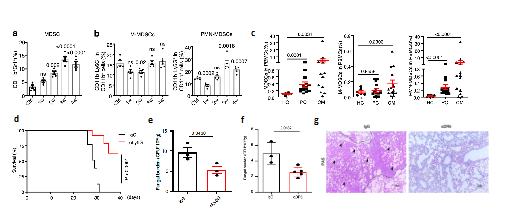
Figure 1 Recruitment of PMN-MDSCs in cryptococcus infection
(Source:Li, et al., Nat Commun, 2022)
Further studies showed that GXM, the main capsular polysaccharide component of Cryptococcus, was the key component to induce the accumulation of PMN-MDSCs, and could inhibit T cell proliferation and function by increasing the expression of Arginase-1 (Arg-1) in MDSCs (Fig. 2a-c). In order to clarify the recognition receptor and downstream signal pathway of GXM, the analysis found that the expression of C-type lectin receptor Clec2d was significantly tuned up after GXM treatment of MDSCs. It was confirmed that Clec2d could specifically recognize GXM in many ways, for example by constructing the fusion protein of Clec2d receptor extracellular segment and human Fc segment (hCLEC2d hFc) and co-culture with Cryptococcus; competitive binding of polysaccharides; binding of Clec2d extracellular segment truncated body; and amino acid site mutation These data confirmed that the expression of Arg-1 in PMN-MDSCs induced by GXM is an important molecule causing an important immunosuppressive effect and the research further discovered the key receptor, Clec2d, that specifically recognizes GXM.
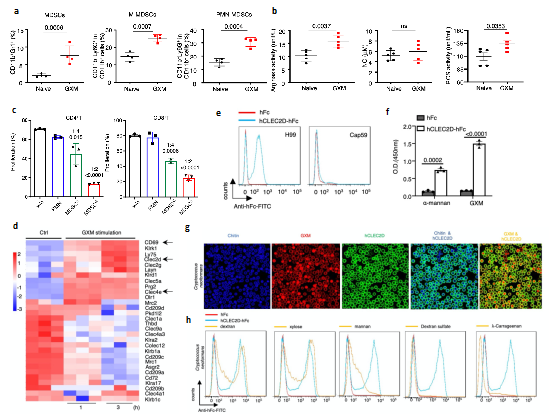
Figure 2 GXM causes PMN-MDSCs to accumulate and be specifically recognized by Clec2d
(Source:Li, et al., Nat Commun, 2022)
To further clarify the intrinsic mechanism of the immunosuppression of Cryptococcus infection caused by GXM, the researchers constructed knockout of Clec2d in mice (Clec2d-/-), and compared the transcriptome of bone marrow-derived MDSCs from wild-type WT mice. KEGG pathway enrichment and GSEA analysis showed that: MAPK pathway changes significantly; p38 pathway activation was inhibited in Clec2d-/- mice; and the use of p38 inhibitor could significantly reduce the expression of Arg-1 in MDSCs (Fig. 3a-e). At the same time, SB202190, a small molecule inhibitor of p38, was used to treat pulmonary Cryptococcus infection in mice, which has significantly improve the survival rate of mice and reduce the amount of bacteria in the lungs. More importantly, the researchers screened 597 non-patented oral drugs and found that vandetanib could inhibit GXM induced p38 pathway activation in vitro. In vivo experiments, it was showed that the drug could also significantly improve the survival rate of mice infected with Cryptococcus; promote the clearance of Cryptococcus; and significantly increase the proportion and function of Th1 and Th17 cells (Fig. 3f-i). The above results indicate that GXM activates the immunosuppressive function of MDSCs through CLEC2D-p38-ARG-1 signal axis. They provide a theoretical basis for the immunotherapeutic strategy of anti-Cryptococcus neoformans infection by intervening p38 pathway to inhibit the expression of ARG-1.
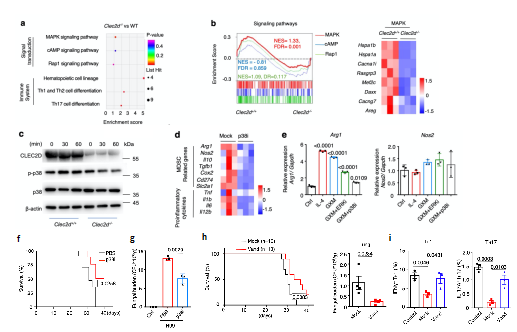
Fig. 3 inhibition of p38 pathway and Arg-1 expression against cryptococcal infection
(Source: Li, et al., NAT commun, 2022)
The control and elimination of Cryptococcus infection mainly depends on the T-cell immune response. Previous studies have shown that Cryptococcus, as an immune inert fungus, causes weak pro-inflammatory responses and immune suppression after infection, resulting in poor treatment of Cryptococcus, especially in immunodeficient patients. Based on clinical cases and animal models, this study revealed that the recruitment and immunosuppressive function activation of PMN-MDSCs induced by GXM are the key factors causing the cryptococcal infection. It further clarified the specific C-type lectin receptor Clec2d and downstream p38-ARG-1 signal axis of MDSCs recognizing GXM. Through small molecule inhibitors and drug screening, it was found that inhibiting this pathway can significantly reduce cryptococcal infection and improve the survival rate of mice. Therefore, it the study provides a new potential immunotherapeutic target and strategy for anti-cryptococcal infection. In conclusion, further exploration in this direction in the future may provide broader applications and ideas for immunotherapy based on MDSCs.
Dr. LI Yanan, assistant researcher of Ruijin Hospital, Dr. WANG Zhongwei, assistant researcher of the Tenth People's Hospital affiliated to Tongji University, LI Fan, doctoral student of Tongji University, and ZHOU LingHong, doctoral student of Huashan Hospital are the co-first authors of this article. The research was supported by the national key research and development program, the National Natural Science Foundation of China and the Shanghai Science and Technology Commission.
Link to article:https://www.nature.com/articles/s41467-022-31723-4