On August 17th, Gao Shaorong from the School of Life Science and Technology of Tongji University and Gao Shuai from China Agricultural University published online a research paper "Inhibition of Wnt activity improves peri-implantation development of somatic cell nuclear transfer embryos" in the National Science Review. This research identified an epigenetic disorder that leads to peri-implantation developmental failure of cloned embryos: abnormal activation of the Wnt signals. More importantly, development of peri- implantation cloned embryos can be significantly improved, thus increasing the birth rate of cloned mice by adding Wnt inhibitors to the culture medium to intervene the Wnt inhibition (Wnti).
As the only way to achieve complete totipotence of somatic cells to date, somatic cell nuclear transfer technology has been widely applied in biology and regenerative medicine. In the past few decades, this technology was successful in over 20 mammalian species, including primates. However, due to various epigenetic obstacles in the reprogramming process of somatic reconstructed embryos, cloned animals had extremely low birth rates and were accompanied by developmental defects in the fetus and placenta. In recent years, researchers have attempted to improve the success rate of nuclear transfer by correcting the abnormal epigenetic modifications established in cloned embryos. Gao Shaorong's research team from the School of Life Science and Technology, Tongji University and Zhang Yi's team from Harvard School of Medicine have identified the mechanisms involved in reprogramming of nuclear transfer embryos, and the barriers to reprogramming caused by key histone modifications (H3K9me3, H3K4me3, and H3K9ac) in pre-implantation embryo development. Effective intervention measures can significantly enhance the potential of nuclear transfer embryos. In addition, abnormal activation of Xist in nuclear transfer embryos and abnormal expression of H3K27me3 dependent imprinting genes can lead to defects in embryonic development after implantation. Restoring their normal expressions through gene editing technology can improve the development and birth rate of cloned embryos after implantation.
However, though the existing methods significantly improved the blastocyst rate and post-implantation development of nuclear transfer technology, the final birth rate was still much lower than that of normal or in vitro fertilized embryos. This means that there are many other unknown barriers that limit the development of cloned embryos. Looking back on the development of nuclear transfer technology, the majority of research focus on exploring development abnormalities in cloned embryos before and after the implantation. Due to the lack of research methods and tools, little is known about the development of cloned embryos at the peri-implantation stage.
The intangible growth of embryos in the mother's uterus at the peri-implantation stage presented great difficulty to research on the development of cloned embryos. How do the formation, signalling and development of cloned embryos happen during this phase? To answer this question, Professor Gao Shaorong's research team has optimized and established a 3D in vitro culture system for SCNT peri-implantation embryos. This system can simulate the peri-implantation development in vivo, achieving its visualization at this stage. It was foundin from vitro culture and in vivo transplantation experiments that most SCNT blastocysts are arrested at the E4.5-E5.25 stage, mainly manifested as significant abnormalities in the transition of inner cell mass (ICM) to outer epiblast (EPI) egg egg cyclinder. The addition of ICM cells from in vitro fertilization embryos during the same period to cloned blastocysts can correct the transformation of the ectoderm and the in vivo implantation rate of embryos, while the addition of ICM cells from cloned embryos has no improvement on the peri-implantation development (Figure 1).
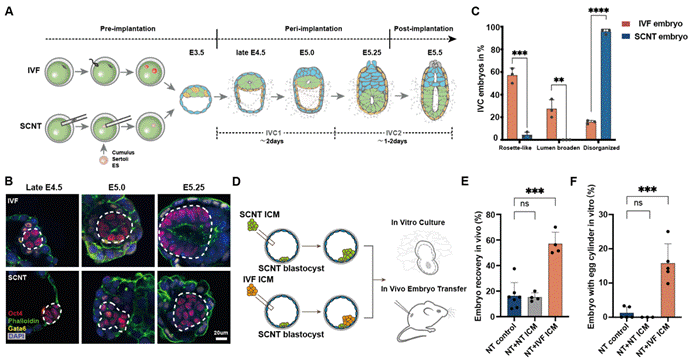
Figure 1: Abnormal formation of the ectoderm of cloned embryos at the peri-implantation stage
To verify the molecular mechanism of EPI developmental abnormalities, researchers conducted extensive/single cell transcriptome analysis on embryos cultured in vitro and in vivo at the peri-implantation stage. The results showed that naïve-to-primed pluripotency transition of EPI from cloned embryos was blocked compared to in vitro fertilized embryos, and that this abnormality generally exists in the development process of cloned embryos from different donor cells (granulosa cells, supporting cells, embryonic stem cells) at the peri-implantation stage. Further analysis of trace histone ChIP-seq data revealed abnormal H3K27me3 deposition in the promoter region of pluripotent genes.
To identify the causes of abnormal pluripotency transition, researchers conducted such experiments as comparative transcriptome, reporting system, and signal intervention, and identified that sustained abnormal activation of Wnt signals is a substantial barrier to the pluripotency transition of cloned embryonic EPI cells. Researchers further discovered that Wnt antagonists, such as Dkk1, Tcf7l1, are significantly inhibited at the pre-implantation blastocyst stage. It was discovered through public data analysis of histone modifications in mouse donor cells and early embryos that H3K27me3 carried by donor cells may be the root of gene expression inhibition of Wnt antagonists such as Dkk1 at the implantation stage (Figure 2).
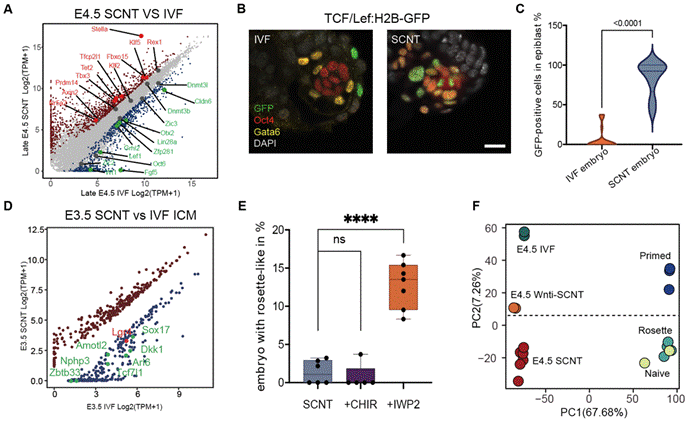
Figure 2: Abnormal activation and intervention of the Wnt in the ectoderm of the peri-implantation cloned embryos
Subsequently, to verify the efficiency of inhibiting the continuous activation of the Wnt at the peri-implantation stage on embryonic development, researchers added the Wnt pathway inhibitor IWP2/IWR1 endo to the culture system to intervene in cloned blastocysts. They found that Wnt pathway inhibitors can significantly promote the transformation of lineage development trajectory, EPI formation, apparent state, and pluripotency state of cloned embryos at the peri-implantation stage (Figure 3). Importantly, cloned blastocysts treated with Wnt pathway inhibitors can significantly improve embryo implantation ability and birth rate after uterine transplantation (the birth rate of nuclear transfer embryos from granulosa cell donors increased from 0.72% to 5.55-7.04%; the birth rate of nuclear transfer embryos from supporting cell donors increased from 0% to 10.3%). Finally, researchers found that the combination of Xist KO and Dnmt3a/b interference can further increase the birth rate of nuclear transfer blastocysts to over 20% by employing previously used epigenetic repair.
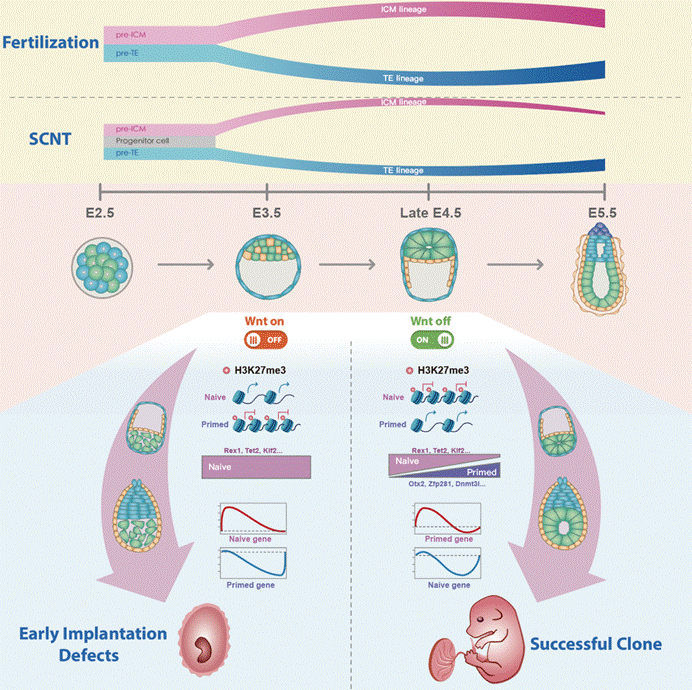
Figure 3: Barrier of abnormal peri-implantation development of cloned embryos
In summary, this research utilized an optimized blastocyst simulation implantation culture system to uncover for the first time the dynamic peri-implantation development of cloned embryos, revealing the biological events of abnormal formation and pluripotency transformation in the ectoderm during this period. Importantly, researchers have found that the sustained activation of the Wnt pathway is an important barrier affecting the peri-implantation development of cloned embryos. Intervention with small molecule inhibitors for Wnt activation and the use of other epigenetic repair can further improve the efficiency of nuclear transfer. This resarch provides important theoretical basis for understanding the peri-implantation development of embryos and expanding the application of cloning technology in developmental biology, regenerative medicine, and animal husbandry.
The first author of the paper is Dr. Li Yanhe from Tongji University and Zheng Caihong, an associate researcher from the Beijing Genome Research Institute. Professors Gao Shaorong, Liu Wenqiang from the School of Life Science and Technology at Tongji University, and Gao Shuai from China Agricultural University are co- corresponding authors. The research was supported by projects funded by the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Shanghai Municipal Science and Technology Commission.